How Potholes Form: Explaining It to Your Clients in Fun Terms
Most drivers who live in cold-weather climates know that they will have to dodge potholes, especially in the spring. They might not, however, understand exactly how potholes form, simply blaming it on road salt and heavy trucks in general.
As a road repair contractor, you probably often receive questions about why potholes happen, and you need to explain it in easy terms without becoming too scientific.
How Potholes Form: The Ice Cube Comparison
Explaining to drivers how potholes form may be as simple as talking to them about an ice cube tray:
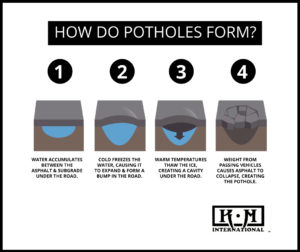
When you fill water into the compartments, the water only goes so high. Once that water freezes, the ice fills the compartment more completely and the tray itself might expand just a little bit. Now, take the tray out of the freezer and allow the ice cubes to melt. You’ll see that each compartment is not as full as it was when it had an ice cube in it.
Now, imagine that you had securely covered the tray with clear plastic wrap after you first filled it with water. Once that water froze, the ice cubes likely caused the plastic wrap to lift up just a little, but when the ice cubes melted, the wrap probably deflated, wrinkled up, and became less secure than it originally was.
Translation: During the winter, water naturally seeps into the ground, freezes, expands, and then melts. Clearly, roadways are stronger than plastic wrap, but when you talk about this process occurring multiple times during the late fall, winter, and early spring season for several years, the roadways will have similar effects. Additionally, the gap between what would be the “plastic wrap” and “melted ice cubes” will allow for even more water to get in – freezing, melting, and further contributing to the roadway damage.
Driving Over the Weakened Spots
When heavy trucks or everyday passenger vehicles regularly drive over these weakened areas of the roadways, material from the roadway can break, creating holes in the ground. The more that vehicles drive over this particular hole, the larger the pothole will become.
Adding Road Salt to the Mix
As much as drivers are grateful for the effects of road salt, it does contribute to how potholes form.
During the dead of a cold winter, the temperatures may not increase past the freezing point of 32 degrees for several days, weeks, or months. That means the temperature on the ground is naturally controlled and the snow can remain in snow form.
Road salt lowers the temperature of the freezing point to about 15 to 17 degrees, and everyday temperatures could be higher, even in the winter. That means water is more likely to freeze and melt more frequently than it would without the application of road salt.
So, contrary to popular belief, it’s not the salt itself that breaks down the integrity of the roads. It’s still about the freeze-thaw cycle.
Side Note: As you’re answering questions from your customers or the general public about how potholes form and the effects of road salt, you might want to add this random fact that they might find interesting:
Road salt only lowers the freezing temperature to about 15 degrees, which is why snow will remain in snow form if you have several days of temperatures below 15 degrees. In other words, the road salt won’t be effective; you won’t get ice because the snow won’t melt; and just during that period of time, the integrity of the roads won’t be sacrificed.
But the spring will eventually come, and that’s when the general public is likely to see your KM International road repair equipment on the roads even more often than in the winter.